| Issue 7 |
The writer Mark Twain once wrote ironically that: "Whiskey is for drinking, water is for fighting". Perhaps there is more than a little truth in this statement. At the Institution of Chemical Engineers AGM in 2007, Sir David King - who at the time was the UK's chief scientific advisor - remarked that the continuing crisis in Darfur, Sudan had been triggered by a dispute over the supply of clean water.
But Darfur isn't the only water-borne crisis. In the Spanish city of Barcelona, water has currently had to be brought in by ship, as extended dry periods have left reservoirs dry. The south-eastern part of Australia, particularly the state of Victoria, is experiencing its worst drought in history. Meanwhile, the increasing use of crops for biofuels drives up demand for irrigation, and global population, along with potable water demand, is increasing rapidly.
Although some 70% of the earth's surface is covered in water, most of that is ocean. In fact, of all the world's water, only 3% is considered 'fresh', and of that quantity, 75% is trapped or locked away in ice caps or glaciers and hence unavailable. The majority of the remainder is stored as groundwater, too deep to be accessible, and only about 1% of fresh water is easily accessible as surface water. In short, aside from some very incremental and expensive gains in overall supply (e.g. through desalination), water remains one of the planet's most precious and finite natural resources.
During the past century, while the population of the world tripled, the use of water increased six-fold. Today, irrigation, industry and municipal use account respectively for 70%, 20% and 10% of total water withdrawals. The high environmental cost of this increased withdrawal is staggering - some rivers no longer reach the sea and many others are at risk (see Table 1). 50% of the world's wetlands have disappeared, 20% of freshwater fish are endangered or extinct, and many of the most important groundwater aquifers are being extracted, with already deep water tables dropping by metres every year, and others already damaged permanently by sea-water ingress. In the Middle East and North Africa region, 85% of water is used for agriculture - much of it for crops that are more easily grown in other places and imported, and nearly 80% of all the water that falls is used. In stark contrast, other areas such as Latin America, the Caribbean and Sub-Saharan Africa use only about 2% of available water.
| ||||||||||||||||||||||||
| Table 1: Summary of threatened rivers (source: WWF, the Environment Agency, the EU Observer) |
1.1 billion people in the world do not have access to clean drinking water, and 2.4 billion lack sanitation, when 80% of developing world diseases are water-borne. Demand for fresh water is increasing. With agriculture using 70% of supply, the World Commission on Water has projected a 60% increase in demand on the water supply to feed two billion more in the next 20 years, and one third of the world population may then be facing a serious water crisis. Compounding the relative scarcity of water is the steady deterioration in water quality in most developing countries. Even in the developed countries, with installed water distribution and purification infrastructures, maintenance of supply is a priority issue against a background of pressure to remediate and reduce pollution. The sustainable supply of potable water and the sustainable treatment of wastewater are thus among the major engineering challenges of the 21st century. Because of this, the response through investment and research is urgent and critical to solving the world's water dilemma.
Recently, the World Bank called for increased investments from private and public sources in order to enhance water security in developing countries. The Bank accounts for about 50% of external financing for water resources - a total of about $3.3 billion a year (19% of all World Bank lending). Their investment portfolio includes water resources management for the environment, water supply and sanitation services, irrigation and hydropower, and the development of related infrastructure. Current investment in new infrastructure in developing and emerging economies is roughly $80 billion per year, but this will have to more than double in the next 20-25 years, to around $180 billion per year. Much of this increase will be for household sanitation, wastewater treatment, treatment of industrial effluents, irrigation and multipurpose schemes, and desalination.
What are the solutions to these challenges? The traditional view of water resource planning has been to predict future needs and develop corresponding infrastructure. In the 1970s, plans for massive reservoirs, big pipelines and river transfers were the best solutions of the day. But today, the environmental impact and energy consumption of such projects are no longer acceptable. Instead, sustainable development of water resources is required, involving technical, socio-economic, political, and institutional issues that underpin sustainable water use. Innovations by engineers and scientists lie at the heart of providing key intellectual and practical solutions to real-world problems of water treatment, supply and resource management. In the words of David King: '... chemical engineers are crucial'.
In June 2003, the Foresight Programme published its report 'Future View' on Sustainable Water Management in the UK. Aimed at key stake-holders, it identified the key driving forces: new regulatory frameworks, the consequences of climate change, and demographic shifts. Sustainability of the UK water sector was a key priority, with emphasis on environmental protection, economic viability and future societal responsibility. The report highlighted innovation as a core-value. It recommended that technology-based innovation should be given significant financial impetus at the research level in new and modified water and wastewater treatment processes, with major energy reduction targets. It also encouraged the take-up of public-funded research in new technology.
Household demand here in the UK is expected to rise by more than 12% in the next 25 years. The current preferred solution is to build five new reservoirs, extend three, and construct two desalination plants to deliver an extra 900 megalitres per day. But water services providers must also look at options to reduce treatment costs, and make better use of existing resources. Options such as real-time control, rainwater harvesting at source, aquifer recharging, and wastewater reuse are cost-effective and sustainable alternatives; the government is now offering capital tax relief on associated equipment, such as membrane filtration. Accurate water metering and pressure monitoring will manage demand and pinpoint water leakage in the distribution network, and water efficient fixtures and appliances (with efficiency labelling) will defer future demand. Integrated pollution control will be better than end-of-pipe treatment for promoting a safer and cleaner environment. To implement this innovation, funding programmes such as the Sustainable Technologies Initiative and investment incentives such as the DTI's Green Technology Challenge will play key roles.
New challenges in the developed world, particularly in Western Europe, North America and Japan, will arise from increasingly stringent legislation on water quality and discharge standards such as the EU Water Framework Directive (see Table 2), emerging environmental issues, and infrastructure improvement. In addition, high rates of growth in potable and wastewater treatment are anticipated in China, Central and Eastern Europe, the Commonwealth of Independent States (formerly USSR), South East Asia and Latin America. Drivers for the latter include long-term economic and industrial development, improvements in the quality of life, and environmental remediation. Future climate change will lead to world-wide droughts and stressed or interrupted supplies on the one hand, and storms and flooding on the other, so that both may eventually threaten economic production and growth. Again, sustainability unites these contradictory drivers. For example, rain harvesting and wastewater recycling cuts down on the use of expensive treated water, and eases pressure on ground water and reservoirs.
| |
| Table 2: Objectives of the EU Water Framework Directive (source: EU) |
Under the direction of Dr Nick Hankins, a University Lecturer in Chemical Engineering in the Department of Engineering Science, a research centre for sustainable water engineering is being developed at Oxford (Figure 1). Its activity focuses on water treatment and supply, and will initially encompass three main areas: potable water treatment and desalination, wastewater treatment and reuse, and industrial process water treatment. A major award of £100,000 from the John Fell Fund main awards scheme in 2007 has provided pump-priming resources for equipping a 100 m2 analytical facility at the Begbroke Science Park. This in turn has allowed the execution of feasibility studies in the three main areas, enabling the preparation of research grant applications. The centre will also play an important part in a major consortium to be established between Singapore, Peking and Oxford universities in environmental resources engineering, by leveraging further resources from the Economic Development Board of Singapore. Past, present and future possible research activities in the three areas are described below.

|
| Figure 1: Oxford Centre for Sustainable Water Engineering |
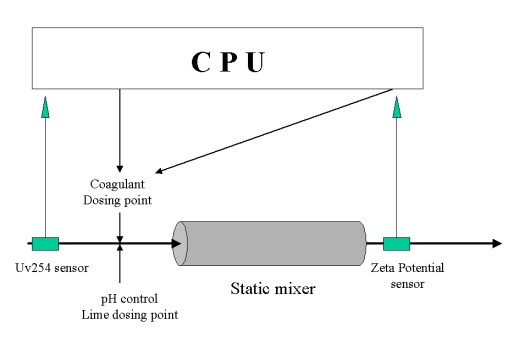
Natural organic matter (NOM) is an increasing source of contamination in drinking water supplies. It is derived from soil humus and terrestrial plants, recent years showing an upward trend in the NOM content of raw water derived from both upland and lowland sources[1]. Traditional 'clarification' treatments based on trivalent, positively-charged cationic coagulants such as aluminium chloride, which cause the negatively charged NOM molecules to aggregate in flocs and settle out (Figure 2), have been relatively successful in the past, but operational problems are now arising and particularly during periods of elevated organic levels during warmer, drier weather. This has an economic impact; the traditional solution has been an increased and excessive safety margin in coagulant dosage. It also has an impact on water quality, with a potential increase in the production of toxic disinfection by-products and a deterioration in taste, odour and appearance. Sustainable improvements in NOM removal strategy are called for, in order to reduce costs and improve removal. On-going work aims to control coagulant costs through real-time NOM monitoring, automatic dosage control, and monitoring of settling. 'Zeta potential', a measure of the negative charge on the NOM, is a highly promising variable for monitoring. A closed-loop dosing process is being developed for use at pilot-plant scale (˜ 1 m3 per day). This consists of a feed forward ultraviolet absorbance sensor to set initial dosage, and a feed-backward zeta sensor for optimisation (Figure 3). Preliminary results have been promising[1], indicating that the combination is superior to the currently used manual control. In another project, sedimentation of the treated water is being analysed using an electrical impedance imaging system developed in the Centre to determine sedimentation rate, which is dependent on dosing and mixing: small aggregates sediment slowly, and lead to large pressure drops in downstream filtration beds.

|
| Figure 2: Solids contact unit for water clarification |
|
| Figure 3: Automated dosage control for potable water clarification |
Osmosis is a naturally occurring, physical phenomenon which has been exploited by human beings since prehistoric times. Early cultures realised that salt could be used to desiccate and disinfect foods for preservation. Possible applications extend from water treatment and desalination, to food processing, power generation, and controlled drug release.
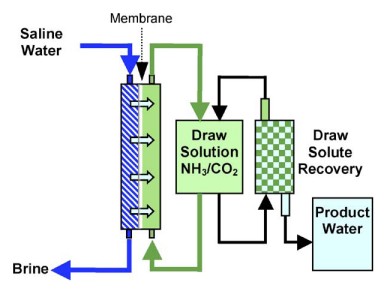
Osmosis is defined as the net movement of water from a weaker, less concentrated solution across a selectively permeable membrane, with rejection of solute molecules or ions, to a stronger, more concentrated solution on the other side. For forward osmosis (FO), the explanation stops there. The more familiar reverse osmosis (RO) uses hydraulic pressure to oppose, and exceed, the osmotic pressure of an aqueous feed solution to produce purified water. But FO employs only the natural osmotic pressure gradient. Water molecules from the feed solution - brackish water or even sea water in unlimited supply - are transported naturally across the membrane by osmosis into a concentrated draw solution on the other side, containing a quite different solute.
As water is drawn from the feed-water across the membrane, the draw solution becomes diluted (Figure 4). In order for the process to be viable, it must be possible to extract the draw solute from it, easily and inexpensively, to leave potable water, and in such a way that the solute can be recycled or otherwise used.
|
|
Figure 4: Forward osmosis process schematic (source: Raphael Semiat and David Hassan: Energy Issues in Desalination Processes, First UK-Israeli Workshop and Research Event on the Application of Membrane Technology in Water Treatment and Desalination, St Hilda's College, Oxford, June 2008) |
Potentially, FO is much more sustainable as a desalination process than RO. The osmotic driving forces in FO can be much greater than the hydraulic driving forces in RO, leading to higher fluxes and recoveries. The use of latent thermodynamic energy and the lack of applied hydraulic pressure makes FO potentially much less energy intensive than RO, while minimising the discharge of brine concentrate. FO has a high rejection of a wide range of contaminants, and may have a lower membrane-fouling propensity.
Finding an effective draw solution is critical to achieving success in a large scale, continuously operated FO system and is the subject of on-going research. An ideal draw solute must have a high osmotic efficiency, high solubility and diffusivity, low toxicity, non-reactivity with the membrane and a low solution viscosity. And it will only be useful if the energy required to separate it is much less than would be required to extract the salt from the original feed solution. Furthermore, new membranes must be developed in both flat-sheet and hollow-fibre configuration, providing high water permeability, high solute rejection, reduced internal fouling, high chemical stability and mechanical strength[2].
A small FO system has already been developed for personal, emergency use, in which sea water can be transformed into a high calorie fruit drink in which the draw solution is concentrated glucose. One such unit consists simply of an immersible bag requiring no electricity or hand pumping.
Wastewater streams contain all the components necessary for sustainability. One m3 of domestic wastewater is normally produced by five to ten people per day and contains enough water for domestic reuse by a similar number of people. It also contains roughly 2 kWh of equivalent chemical energy, which covers the domestic power needs of one person for half a day in developed countries (and ten people for a day in developing countries!) and enough nutrients to fertilise 1 m3 of agricultural production for a year. At a glance, it can be seen that recovery of these resources from wastewaters could have a dramatic global impact[3].
Yet current treatment plants actually consume additional energy to destroy the organic compounds and nutrients available, and much of the treated water is not even reused! Wastewater treatment processes currently represent 3-5% of the total electricity consumption in developed countries. The remaining energy and nutrients in the sludge are increasingly incinerated and buried in landfills, rather than being used as fertilisers! Clearly such practices are not sustainable.
We must change to an attitude in which we see wastewater treatment as an exercise in resource recovery. To reduce water resource demands, membrane processes are being investigated to recycle water for both non-potable and potable uses. One of the leaders in this field is the small nation of Israel, which recycles 60-65% of its wastewater. Promising water technology companies there may turn Israel into the water technology equivalent of Silicon Valley, with $5 billion in exports by 2010.
Relatively simple and highly sustainable concepts are appropriate, applying Natural Biological Mineralisation Routes (NMBR) for wastewater treatment, and implementing Decentralised Sanitation and Resource Recovery and Reuse (DESAR). The former implies the use of a biological degradation sequence of organic residues under natural conditions involving anaerobic digestion followed by further aerobic processes as a post-treatment. The latter implies transport of wastewater is kept to an optimum level and that use is made of the extracted pollutants.
To recover energy from wastewater streams, a number of options are available and are increasingly the subject of research efforts. The reduction of energy and chemical inputs can be achieved by changing to an anaerobic biological treatment, or from long to short sludge-age aerobic processes and using biological rather than chemical phosphorus removal. Methane recovery is possible through anaerobic processes, particularly for high strength wastewaters, using the methane to replace natural gas. Energy conversion efficiencies are potentially much higher than for bio-fuels, and might allow treatment plants to be more than energy self-sufficient.
Perhaps even more promisingly, microbial fuel-cells - combined bioreactors and fuel cells - are gaining major attention for direct electricity generation, and are suited to low concentration, low strength wastewaters at ambient temperatures. They generate electricity in one step, no gas treatment is necessary, and effluent quality is better than for anaerobic processes. Nevertheless, challenges remain in scale-up and the large capital costs - this technology has its real niche in places where electricity is very precious!
Finally, the chemical energy in wastewater may be converted to value added products, either by direct conversion of the organics into products, like biopolymers, or by using electrons from the organics to generate new products such as hydrogen, ethanol or 1,3 propanediol.
The contamination of freshwater systems with an increasing number of industrial and natural chemical compounds is a key environmental problem. Although they are present at low concentrations, such micro-contaminants raise significant toxicological problems. Such chemicals do not degrade (e.g. heavy metals) or only very slowly (e.g. DDT or polychlorinated biphenyls). Cost effective and appropriate remediation and water treatment technologies must be developed and refined. An example is the rising levels of endocrine (hormonal) disrupting compounds, such as oestrogen, discharged from sewage treatment works into the environment, an issue of increasing concern to the UK Environment Agency. This is not least because they have the potential to interfere with the normal reproduction and endocrine systems of aquatic organisms. We are currently investigating ways to increase oestrogen and other micro-contaminant removal efficiency by sewage treatment works above 90% by practical and cost-effective improvements to existing plants.
The process industries are under increasing pressure to reduce the discharge and contaminant level of aqueous effluents; rather, they must recycle water associated with internal streams, and recover from them valuable product intermediates. Conventional physico-chemical technologies for pollutant removal exist in water treatment; when the additional need to recycle the pollutant is considered, they face severe drawbacks, such as cost, energy usage and mineralisation. Amongst novel and promising methods to achieve this, methods based on surface-active agents (surfactants, often found in soaps and cleaning agents) have received strong attention in recent years: aggregates called micelles form in aqueous solutions and provide a versatile and powerful environment for the removal of dissolved pollutants.
A novel colloidal flocculation process known as Adsorptive Micellar Flocculation (AMF) has been investigated at the bench-scale[4]. When negative anionic surfactant ions form micelles in aqueous solution, strongly charged positive cations are then able to bind on to the surface of the micelles. The charge neutralised micelles are thus made to 'flocculate' or aggregate. Simultaneously, if the charged anion of a dissociated organic acid pollutant (a process end product or synthetic intermediate) such as the pesticide 2,4-D is present, it will associate locally and strongly with the highly positive charge of the bound multivalent cations. The anionic pollutant remains associated with the bound cations within flocculated micelles. Because of this association with the micelles, the anionic pollutant is effectively removed from the bulk aqueous solution upon flocculation. The resulting macroscopic 'flocs' retain the anionic pollutants within their structure. Since they have a rather open, porous structure, they are easy to filter off on to paper or cloth, while the purified bulk water can be drained off rapidly. The flocs can be dried, and pollutant then separated from surfactant and flocculant by solvent extraction; the surfactant and flocculant is recycled to the AMF process, and the concentrated pollutant is either recycled to the process upstream or destroyed.
Previous laboratory-scale work has provided substantial insight into AMF in terms of removing a variety of industrial aqueous effluents, including organic acid pesticides such as 2,4-diphenoxy-acetic acid and synthesis intermediates (benzoic acid, phenol, phthalic acid). An investigation into the types of aqueous streams suitable as targets shows that AMF is particularly effective on solutions of organic acids and bases, especially those with a degree of aromacity. These illustrate the great potential of AMF as a sustainable, industrial process stream treatment technique.
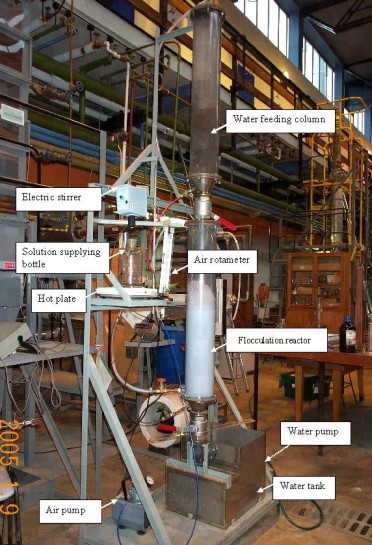
The process has the advantage of simplicity, efficiency, low cost and low volume. In order to further develop the application of this promising water treatment technique, pilot feasibility studies (Figure 5) are essential for establishing the scale-up from the bench-scale to a continuously-operated unit at commercial scale, focussing on flocculation time, floc settling, optimum agitation rate and reagent dosage. Residual concentrations in treated water indicate a surfactant removal efficiency of 95-98%, and pollutant removal/recycle reaches 78% in two stages of process operation. If reagent recycle is employed according to a previously developed strategy, the process may prove to be economically viable.
|
| Figure 5: Pilot Plant for Adsorptive Micellar Flocculation |
We all know bottled water is big, if not sustainable, business. But now Holy Drinking Water is being filtered and bottled in the San Joaquim valley, California, taking things to a divine level. Though it is normally associated with a church, Brian Germann of Linden decided to bottle it. He got the idea on 6 June 2006 - 6.6.6. He asked himself "what if you could drink holy water as a defence against evil?".
He found a company in Stockton willing to do the bottling, and took on some clergymen willing to perform the blessing. The warning to sinners printed on the label says: "If you are a sinner or evil in nature, this product may cause burning, intense heat and oral irritations".
References
[1] Price R and Hankins N "Impact of Optimised Control of the Pre-Coagulation Regime During Ultra-filtration Treatment of Raw Upland Waters". Water and Environment Journal. 2005. 19(4), 342-351.
[2] Cath TY, Childress AE and Elimelech M: "Forward Osmosis: Principles, Applications and Recent Developments". Journal of Membrane Science. 2006. 281, 70-87.
[3] Keller J: "Reduce, Recover, and...?", The Chemical Engineer, June 2008, 24-25.
[4] H Sun, NP Hankins, BJ Azzopardi, N Hilal and CAP Almeida: "A Pilot-Plant Study of the Adsorptive Micellar Flocculation Process: Optimum Design and Operation", Separation and Purification Technology. 2008, 62, 273-280.
| << Previous article | Contents | Next article >> |
| SOUE News Home |
Copyright © 2008 Society of Oxford University Engineers |
SOUE Home |